On Wednesday, the Gale Group enjoyed pizza and bowling at the Student Union. A fun time was had by all!


News
On Wednesday, the Gale Group enjoyed pizza and bowling at the Student Union. A fun time was had by all!


In December, the Gale Group gathered to celebrate the end of the year with a Christmas party. Featuring a potluck, a white elephant gift exchange, and lively conversation, the event provided a great opportunity to relax outside the lab and strengthen connections.

Munawar Jawad et al. recently published “A disposable, passive microfluidic cartridge for point-of-care detection of antibodies in total capillary blood based on hemagglutination and machine-learning assisted interpretation” in RSC Advances.
Abstract: Point-of-care (PoC) detection of antibodies in blood enables rapid, on-site diagnosis. However, these devices often face challenges related to user variability due to the requirement of multiple manual operations. To address this issue, we designed and developed a disposable microfluidic device that requires minimal user input for rapid detection of SARS-CoV-2 antibodies (ABs) in total blood and antigens associated with blood types. Here, we present a passive pressure-driven pumping technique that rapidly mixes blood samples with reagents, delivering results within three minutes. The device requires 15 mL of capillary blood and can detect SARS-CoV-2 ABs across a concentration range of 0 to 60 mg mL−1. Additionally, we demonstrated the versatility of the microfluidic device by implementing blood typing functionality, highlighting its potential for broader serological testing applications. We also developed a support vector machine (SVM) algorithm as a proof-of-concept to demonstrate the potential application of machine learning (ML)-based analysis to complement visual interpretation of results. We evaluated the performance and predictive accuracy of the SVM model and compared it to human interpretations. The analysis showed that the SVM model achieved a statistically significant improvement in predicting varying degrees of agglutination when compared to human interpretation. This device addresses the need for a user-friendly, rapid COVID-19 AB testing solution and blood-typing assay and also provides a model for the future development of diagnostic devices that are integrated with ML models for improved diagnostic accuracy and accessibility in both clinical and non-clinical environments.
A figure of the hemagglutination assay is displayed and the full article can be found at https://doi.org/10.1039/D5RA05719A.

The Gale Group enjoyed a game of pool at the Student Union building this past Friday.

This Friday, the Gale Group enjoyed lunch at Ruth’s Diner and the beautiful fall weather.

Congratulations to the following students from the Gale Group who have successfully defended and/or graduated within the past year!
Yunhao Peng
Greg Liddiard
Afroza Tabassum Akhi
Shamima Juthi
Sabin Nepal
Matt Nelson

Brady Goenner et al. recently published “An open source platform to automate the design, verification, and manufacture of 3D printed microfluidic devices” in Scientific Reports.
Several barriers to widespread microfluidic adoption exist, including high initial fabrication costs and the labor-intensive development process. To address these barriers, a toolchain for the design, verification, and manufacturing of 3D printed microfluidic devices is presented. This work builds on existing electronic design automation (EDA) tools, yielding a toolchain that automatically lays out a microfluidic device from a library of components, simulates the device, and produces a 3D CAD file for manufacture via 3D printing. The process is validated by automatically designing and fabricating a calcium quantification assay. The full article can be found at https://doi.org/10.1038/s41598-025-15976-9.
This past Friday, the Gale Group hiked Gloria Falls up Little Cottonwood Canyon. It was a great group activity to kick off the semester and enjoy the end of summer in Utah!

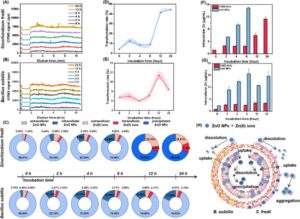
“Real-time monitoring of culture medium- and species-specific transformation of zinc oxide nanoparticles in bacterial systems using coupled CyElFFF-ICPMS,” authored by Weichen Zhao et al., was recently accepted in Microchemical Journal.
The article outlines the use of an online-coupled cyclical electrical field-flow fractionation and inductively coupled plasma mass spectrometry (CyElFFF-ICPMS) system for the quantification and characterization of the promising nanoagrochemicals of zinc oxide nanoparticles (ZnO NPs) and Zn(II) ions. Using the CyElFFF-ICPMS system, bacillus subtilis is found to stabilize ZnO NPs while sinorhizobium fredii promotes extracellular ZnO NP aggregation and dissolution. After 6 h, intracellular transformation significantly diverges. These findings demonstrate that the stability, transformation, and intracellular fate of ZnO NPs are highly governed by bacterial species-species interactions. The intracellular transformation and distribution of ZnO NPs in bacterial systems is displayed in the figure and the full article can be found at https://doi.org/10.1016/j.microc.2025.114926.