Publication on a Disposable, Passive Microfluidic Cartridge for Antibody Point-of-Care Detection
Munawar Jawad et al. recently published “A disposable, passive microfluidic cartridge for point-of-care detection of antibodies in total capillary blood based on hemagglutination and machine-learning assisted interpretation” in RSC Advances.
Abstract: Point-of-care (PoC) detection of antibodies in blood enables rapid, on-site diagnosis. However, these devices often face challenges related to user variability due to the requirement of multiple manual operations. To address this issue, we designed and developed a disposable microfluidic device that requires minimal user input for rapid detection of SARS-CoV-2 antibodies (ABs) in total blood and antigens associated with blood types. Here, we present a passive pressure-driven pumping technique that rapidly mixes blood samples with reagents, delivering results within three minutes. The device requires 15 mL of capillary blood and can detect SARS-CoV-2 ABs across a concentration range of 0 to 60 mg mL−1. Additionally, we demonstrated the versatility of the microfluidic device by implementing blood typing functionality, highlighting its potential for broader serological testing applications. We also developed a support vector machine (SVM) algorithm as a proof-of-concept to demonstrate the potential application of machine learning (ML)-based analysis to complement visual interpretation of results. We evaluated the performance and predictive accuracy of the SVM model and compared it to human interpretations. The analysis showed that the SVM model achieved a statistically significant improvement in predicting varying degrees of agglutination when compared to human interpretation. This device addresses the need for a user-friendly, rapid COVID-19 AB testing solution and blood-typing assay and also provides a model for the future development of diagnostic devices that are integrated with ML models for improved diagnostic accuracy and accessibility in both clinical and non-clinical environments.
A figure of the hemagglutination assay is displayed and the full article can be found at https://doi.org/10.1039/D5RA05719A.

Publication on an Open Source Platform to Automate the Design, Validation, and Manufacture of Microfluidics

Brady Goenner et al. recently published “An open source platform to automate the design, verification, and manufacture of 3D printed microfluidic devices” in Scientific Reports.
Several barriers to widespread microfluidic adoption exist, including high initial fabrication costs and the labor-intensive development process. To address these barriers, a toolchain for the design, verification, and manufacturing of 3D printed microfluidic devices is presented. This work builds on existing electronic design automation (EDA) tools, yielding a toolchain that automatically lays out a microfluidic device from a library of components, simulates the device, and produces a 3D CAD file for manufacture via 3D printing. The process is validated by automatically designing and fabricating a calcium quantification assay. The full article can be found at https://doi.org/10.1038/s41598-025-15976-9.
Publication on the Use of CyElFFF-ICPMS for the Quantification and Characterization of Zinc Oxide Nanoparticles
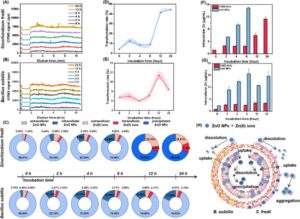
“Real-time monitoring of culture medium- and species-specific transformation of zinc oxide nanoparticles in bacterial systems using coupled CyElFFF-ICPMS,” authored by Weichen Zhao et al., was recently accepted in Microchemical Journal.
The article outlines the use of an online-coupled cyclical electrical field-flow fractionation and inductively coupled plasma mass spectrometry (CyElFFF-ICPMS) system for the quantification and characterization of the promising nanoagrochemicals of zinc oxide nanoparticles (ZnO NPs) and Zn(II) ions. Using the CyElFFF-ICPMS system, bacillus subtilis is found to stabilize ZnO NPs while sinorhizobium fredii promotes extracellular ZnO NP aggregation and dissolution. After 6 h, intracellular transformation significantly diverges. These findings demonstrate that the stability, transformation, and intracellular fate of ZnO NPs are highly governed by bacterial species-species interactions. The intracellular transformation and distribution of ZnO NPs in bacterial systems is displayed in the figure and the full article can be found at https://doi.org/10.1016/j.microc.2025.114926.
Publication on an Osmosis-Driven Brain Implant for Drug Delivery
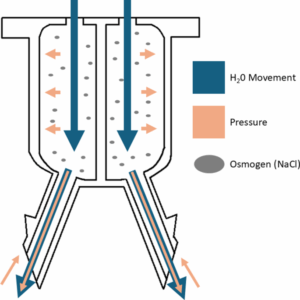
Ata Ullah and Jade Bookwalter et. al recently published “An Osmosis-driven 3D-printed brain implant for drug delivery” in Biomedical Microdevices.
To combat glioblastoma, a highly malignant brain tumor, several different drug-loaded devices have been developed to suppress tumor recurrence. However, these implants have limited effectiveness and often fail due to clogging, reflux, and limitations in intracranial implantation. Therefore, this article outlines the design, fabrication, and results of an osmosis-driven, 3D-printed brain implant. Featuring dual reservoirs, osmotic membranes, and precision-engineered needles, the implant achieves flow rates of 2.5±0.1 µl/Hr and diffusion distance up to 15.5±0.4 mm. A schematic of the working principle of the device is displayed in the figure and the full article can be found at https://doi.org/10.1007/s10544-025-00759-w.
Publication on the Isolation and Removal of Round Spermatids from a Spermatogenic Cell Sample Using Microfluidics
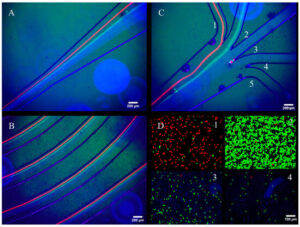
“Application of Inertial Microfluidics for Isolation and Removal of Round Spermatids from a Spermatogenic Cell Sample to Assist In-Vitro Human Spermatogenesis” authored by Sabin Nepal, Joey Casalini, Alex Jafek, and Bruce Gale was recently published in Micromachines.
The article outlines the used of inertial microfluidics for isolating round spermatids from other germ cells and purifying spermatogenic cells as a way of improving in-vitro spermatogenesis to address male infertility. A custom PDMS microfluidic spiral channel for performing separation is designed, fabricated, and tested. The custom device does not experience clogging issues, a problem encountered in a commercially available spiral device. Additionally, the fabricated device achieves 86% purity in a single pass, an improvement over the 38% seen with STA-PUT – a method based on velocity sedimentation commonly used in this application. Validation results of the fabricated device are shown in the figure with the full article being found at https://doi.org/10.3390/mi16050500.
Publication on Protozoan Parasite Monitoring System
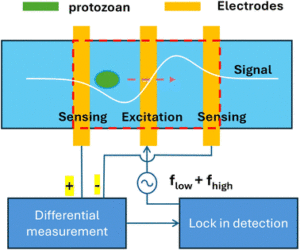
Authored by Yunhao Peng, Bruce K. Gale, and Himanshu J. Sant, “Waterborne protozoan parasite detection using two-frequency impedance flow cytometry” was recently published in Analytical Methods.
A common cause of gastrointestinal diseases, waterborne parasitic protozoa are micron-sized parasites present in water sources. Therefore, the article outlines the development of a microfluidic water monitoring system based on impedance flow cytometry for the detection of these parasites. By utilizing parallel rather than coplanar electrodes, a limit detection of <0.1% volume ratio is achieved. Additionally, to improve sample discrimination, both a low and high frequency are applied simultaneously, making the method outlined in the article distinct from other proposed systems. A schematic of the monitoring system is displayed in the figure and the full journal article can be found at https://doi.org/10.1039/D5AY00184F.
Publication on a Spiral Channel with Integrated Microelectrodes for Particle Lateral Position and Size Characterization
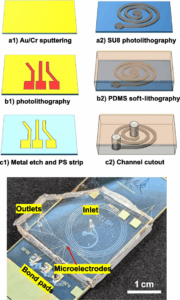
“A spiral channel with integrated microelectrodes for label-free particle lateral position and size characterization,” authored by Yunhao Peng, Bruce K. Gale, and Himanshu J. Sant, was recently published in Biomedical Microdevices.
In the article, a spiral-shaped microfluidic channel integrated with modified-trident shaped microelectrodes is utilized to analyze and quantify separation of different sized particles. Lateral particle position corresponds to the ratio of peak amplitudes, while peak amplitude indicates particle size and vertical position. The device yields a particle size estimate sensitivity of 2.15 µm/mV. The device fabrication process is displayed in the figure and the full journal article can be found at https://doi.org/10.1007/s10544-025-00742-5.
Publication on a Trident-Shaped Electrode Design for Particle Lateral Position Detection
Yunhao Peng et al. recently published an article entitled “Modified trident-shaped electrode design for particle lateral position detection in microfluidic impedance flow cytometry” in Sensors and Actuators: A. Physical.
The article outlines the use of modified trident electrodes inside a microfluidic channel for the implementation of impedance flow cytometry for the detection of single particles and their lateral positions. The proposed design is displayed in the Figure below. The device is shown to detect impedance differences caused by less than 0.02% volume displacement. Additionally, using amplitude values, two different-sized microspheres were identified while showing an increase in signal amplitude with a smaller distance between electrodes. The full article can be found here: https://doi.org/10.1016/j.sna.2024.116062.
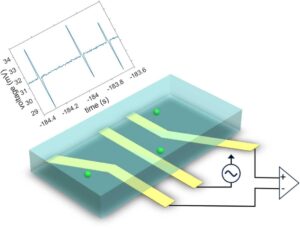
Figure. Illustration of the proposed trident-shaped electrode for the detection and measurement of lateral particle locations (y) of flowing particle/cells inside a microfluidic channel.
Automating the Microfluidic Design Process
 Professor Bruce Gale, Ph.D., Department of Mechanical Engineering Chair and Merit Medical Professor at University of Utah, uses microfluidics to solve problems. In the past he has developed tools for drug development, pathogen detection, fast PCR technologies, medical devices and more. Now he is turning that expertise toward automating the design of microfluidic devices to perform specific tests, assays, and more.
Professor Bruce Gale, Ph.D., Department of Mechanical Engineering Chair and Merit Medical Professor at University of Utah, uses microfluidics to solve problems. In the past he has developed tools for drug development, pathogen detection, fast PCR technologies, medical devices and more. Now he is turning that expertise toward automating the design of microfluidic devices to perform specific tests, assays, and more.
Gale received two million dollars in funding from NSF’s LEAP HI program to pursue “Microfluidic Design Automation for Biomedical Assays.” He will be working with associate professor of Electrical & Computer Engineering Pierre-Emmanuel Gaillardon to take advantage of existing software that has been created for laying out computer chips and adapting it for microfluidics. He will also partner with a lab at BYU that has a lot of expertise in 3D printing microfluidic devices, taking advantage of building devices that work in three dimensions.
“One of the biggest problems in microfluidics is that every time someone wants a device, they need to hire an expert to design and build the device,” said Gale. “There really isn’t a standard way to make a cheap device at intermediate scale. A lot of applications don’t require millions of parts. If you only need ten or a hundred thousand devices, you don’t get the benefits of mass production. This approach could solve that problem.”
The program Gale intends to develop would allow a doctor/clinician/ or other individual needing a test or device to enter a basic summary of the needed protocol. The program would then use that to generate a CAD layout for the microfluidic chip and run a simulation to show that it works as intended. From there it could 3D print the chip. This could take the entire process of creating a new microfluidic device down to a couple of hours or even less.
Gale also sees this research increasing accessibility to microfluidics. He wants students to have the opportunity to create their own microfluidic devices. By offering a digital process with more automation, it will be much easier than generating these tools by hand. Gale also wants to create a store that has available finished modules that could be used as is or used to help generate additional designs, and that could be printed on-demand.
“This could help out small facilities, like those you’d find in rural Utah,” said Gale. “They don’t have to keep an inventory of devices around or buy things you may not need. You just download or generate the specific devices you need when you need them.”
This increase in access and production capabilities will help drive down prices and make these devices more readily available, even extending to printing tests at home.
“If I do this well, I’ll put myself out of business,” Gale joked.
You can learn more about Dr. Gale’s work through his lab website.
